Main Difference – Protium vs Deuterium vs Tritium
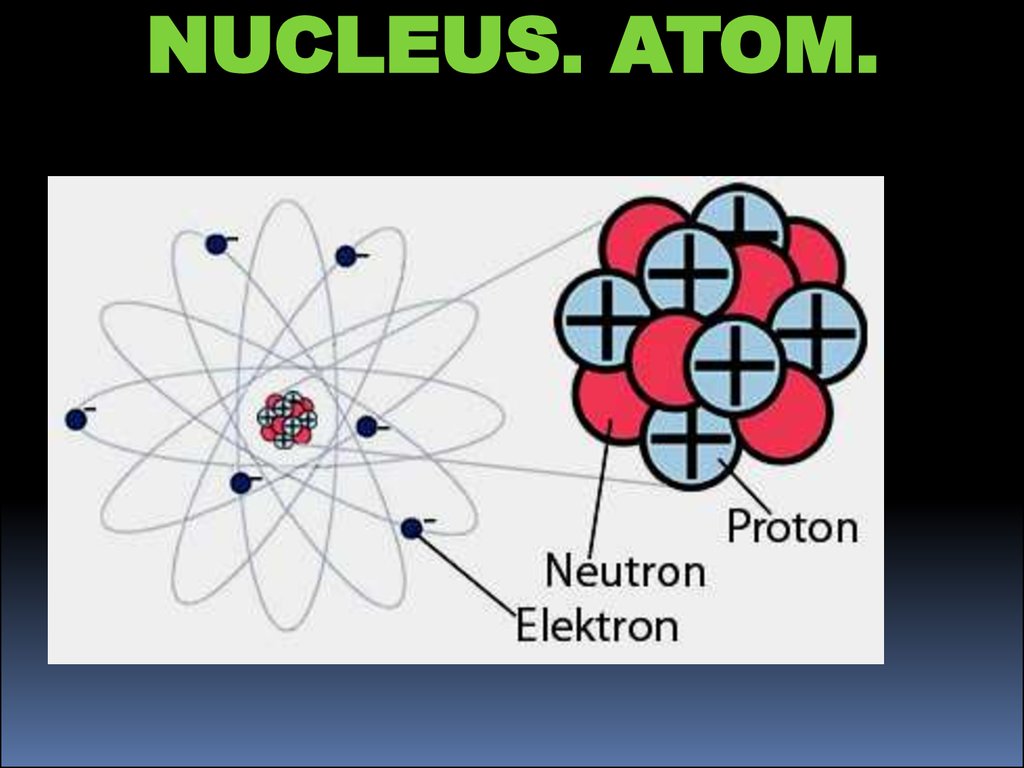
Protium, Deuterium, and Tritium are isotopes of the Hydrogen element. Isotopes are different forms of the same element that are different from each other according to the number of neutrons they have in their nuclei. Therefore, isotopes have the same atomic number but different atomic masses. Due to this reason, isotopes have different physical properties but the chemical properties remain the same because the number of electrons present in isotopes is equal. Therefore Protium, Deuterium, and Tritium share some similarities as well as differences. The main difference between Protium Deuterium and Tritium is that Protium has no neutrons in its nuclei whereas Deuterium is composed of one neutron and Tritium is composed of two neutrons.
Key Areas Covered
1. What is Protium
– Definition, Properties, and Abundance
2. What is Deuterium
– Definition, Properties, and Abundance
3. What is Tritium
– Definition, Properties, and Abundance
4. What are the Similarities Between Protium Deuterium and Tritium
– Outline of Common Features
5. What is the Difference Between Protium Deuterium and Tritium
– Comparison of Key Differences
Key Terms: Atomic Mass, Atomic Number, Deuterium, Isotopes, Neutron, Protium, Tritium
What is Protium
Protium is an isotope of Hydrogen that is composed of one proton and one electron. It is the most abundant form of hydrogen. The abundance of this isotope in the earth’s crust is about 99.9%. Protium has no neutrons in its nucleus. It is considered as the most stable isotope of hydrogen. Therefore, when we are normally talking about hydrogen, we are talking about Protium.
The atomic number of Protium is 1 due to the presence of one proton. The mass number of Protium is also 1 since there are no neutrons in the nucleus of Protium. The atomic mass of Protium is about 1.00794 amu. The symbol for Protium is 1H. The electron configuration of Protium is 1s1.
It has a natural abundance of 156.25 ppm in the oceans, and accounts for approximately 0.0156% of all hydrogen found on earth. The nucleus of deuterium, called a deuteron, contains one proton and one neutron (mass number = 2), whereas the far more common hydrogen isotope, protium, has no neutrons in the nucleus. The atomic number of deuterium is one. This is because deuterium is an isotope of hydrogen, thus it has the same atomic number as hydrogen. Deuterium, (D, or 2H), also called heavy hydrogen, isotope of hydrogen with a nucleus consisting of one proton and one neutron, which is double the mass of the nucleus of ordinary hydrogen (one proton). Deuterium has an atomic weight of 2.014. It is a stable atomic species found in natural hydrogen compounds to the extent of about 0.0156 percent. Deuterium Oxide is a stable, non-radioactive isotopic form of water, containing 2 atoms of deuterium (D) and one atom of oxygen (2D2O), with DNA-labeling activity. Upon ingestion of deuterium oxide, 2H is incorporated into the deoxyribose moiety of DNA of newly divided cells. Rapidly dividing cells, as in the case of B-cell chronic lymphocytic leukemia (B-CLL), can be labeled with deuterium. It is not on the periodic table! Deuterium is simply Hydrogen with one extra neutron (i.e., 1 proton, but the same atomic weight as He). Its natural abundance (which is virtually nothing) is factored into the average atomic weight of H which as you notice is NOT exactly 1 amu).
Protium can be found in nature as a diatomic gaseous form or as hydrogen in H2O molecule. The bond between two atoms in the diatomic molecule has a higher bond dissociation enthalpy. This is mainly because these atoms are minute and they have complete electron configurations in the only orbital (s orbital) in their diatomic molecule form.
The above image shows the atomic structure of Protium. Here, the proton is shown in the center of the atom (nucleus) and the electron is shown outside the nucleus in blue color.
What is Deuterium
Deuterium is an isotope of Hydrogen that is composed of one proton, one neutron, and one electron. The nucleus of Deuterium is composed of a proton and a neutron. The symbol for Deuterium is given as 2H. The atomic number of Deuterium is 1 and the mass number is 2. The atomic mass can be given as 2.014 amu. This is also a stable isotope of hydrogen but is less abundant. The abundance of Deuterium in the earth’s crust has been calculated as 0.015%. It is not radioactive since Deuterium is stable with one proton and one neutron in its nucleus.
The occurrence of Deuterium can be either in gas phase or liquid phase. Deuterium exist as diatomic gases such as D2 or HD (in combination with hydrogen). If not, Deuterium can be found as heavy water. Heavy water is composed of D2O molecules. Most of the times, Deuterium act in a similar manner as Protium. But there are certain differences too. Due to the presence of the neutron, the atomic mass of Deuterium is as twice as Protium. Therefore, the bond length and bond energy are different from those of Protium. Moreover, ice made from heavy water will sink in liquid water due to high density (normal ice floats on liquid water surface).
There are some applications of Deuterium as well. In NMR spectroscopy, Deuterium included compounds are used as the solvent instead of compounds composed of Hydrogen. Then, the peaks given by hydrogen atoms of the analyte can be distinguished by the atoms of the solvent.
What is Tritium
Tritium is an isotope of hydrogen that is composed of one proton, two neutrons, and one electron. The symbol for Tritium is 3H. The atomic number of Tritium is 1 and the atomic mass of Tritium is 3. The mass can be given as 3.016 amu. This isotope of hydrogen is radioactive due to the presence of a high number of neutrons compared to the number of protons.
Tritium often undergoes beta decay. This produces Helim-3 and it releases a large amount of energy. The half life of Tritium has been calculated as 12.32 years. However, the abundance of Tritium on earth’s crust is very less.

Figure 3: Atomic Structure of Tritium
The above image shows the atomic structure of Tritium. The mass number of Tritium is 3 due to the presence of two neutrons (in red color) and a proton (in blue color).
Similarities Between Protium Deuterium and Tritium
- Protium, Deuterium and Tritium are isotopes of hydrogen.
- These isotopes are composed of 1 proton per nucleus.
- All three are composed of 1 electron.
Difference Between Protium Deuterium and Tritium
Definition
Protium: Protium is an isotope of Hydrogen that is composed of one proton and one electron.

Deuterium:Deuterium is an isotope of Hydrogen that is composed of one proton, one neutron, and one electron.

Tritium:Tritium is an isotope of hydrogen that is composed of one proton, two neutrons, and one electron.
Abundance
Protium:The abundance of Protium is about 99.9%.
Deuterium: The abundance of Deuterium is about 0.015%.
Tritium: Tritium is found in very trace amounts.
Chemical Symbol
Protium: The symbol for Protium is 1H.
Deuterium: The symbol for Deuterium is 1H.
Tritium:The symbol for Tritium is 1H.
Mass Number
Protium: The mass number of Protium is 1.
Deuterium:The mass number of Deuterium is 2.
Tritium: The mass number of Tritium is 3.
Atomic Mass
Protium: The atomic mass of Protium is 1.00794 amu.
Deuterium: The atomic mass of Deuterium is 2.014 amu.
Tritium: The atomic mass of Tritium is 3.016 amu.
Radioactivity
Protium: Protium is not radioactive.
Deuterium Atomic Number And Mass Number
Deuterium:Deuterium is not radioactive.
Tritium: Tritium is radioactive.
Conclusion
Protium, Deuterium and Tritium are three isotopes of hydrogen. Apart from these isotopes, there can be some other forms of hydrogen as well. But they are highly unstable due to the presence of a high number of neutrons. The main difference between Protium Deuterium and Tritium is that Protium has no neutrons in its nuclei while Deuterium is composed of one neutron and Tritium is composed of two neutrons.
Deuterium Element
References:
1.”Isotopes of Hydrogen – Boundless Open Textbook.” Boundless. Boundless, 20 Sept. 2016. Web. Available here. 02 Aug. 2017.
2.”Deuterium, Tritium, and Protium – Three Hydrogen Isotopes.” Quirky Science. N.p., 25 Mar. 2017. Web. Available here. 02 Aug. 2017.
Image Courtesy:
1. “Hydrogen” By Mets501 – Own work (CC BY-SA 3.0) via Commons Wikimedia
2. “H-2 atom” By ZYjacklin – Own work (CC0) via Commons Wikimedia
3. “Blausen 0528 Hydrogen-3 Tritium” By BruceBlaus – Own work (CC BY 3.0) via Commons Wikimedia
4. “204 Isotopes of Hydrogen-01” By OpenStax College – Anatomy & Physiology, Connexions Web site. Jun 19, 2013. (CC BY 3.0) via Commons Wikimedia
In the nuclear symbol for deuterium, #'_1^2H#, what is the atomic number and the mass number?
1 Answer
Explanation:
Nuclear Symbol For Deuterium Atomic Number
And since it is an hydrogen isotope we can IMMEDIATELY say that
And
The mass number is simply the number of
Related questions
